Introduction
Ataxia is a medical condition which is characterized by various coordination disturbances due to damage to the cerebellum (part of the brain that regulates motor function and coordination), the spinal cord or peripheral neurons. It can develop at any age depending on its cause. The condition may accompany diseases affecting the nervous system, such as stroke, immunologic diseases (including multiple sclerosis and celiac disease), neoplasms, infections, alcoholic degeneration, etc. There are also several hereditary forms caused by gene mutations.
Identification of the underlying condition is the focus and the main challenge in ataxia management. The diagnosis is determined based on medical and neurological history, CT or MRI of the brain to reveal changes in the cerebellum, and blood tests that can reveal vitamin or coenzyme deficiencies.
Treatment of ataxia is challenging due to the vast array of potential causes. Current clinical studies explore new medications as well as novel approaches, including stem cell treatment.
Symptoms and Common Signs of Ataxia
Patients with ataxia may experience problems with daily activities and independent living skills. They usually first notice problems with coordination, which may manifest as gait impairment and frequent stumbling. These initial problems with walking may be later accompanied with the following signs and symptoms:
- hesitation in speaking;
- problems with handwriting and typing;
- difficulty with chewing and swallowing;
- vision and cognitive dysfunctions;
- fatigue;
- difficulty with concentration;
- heart problems;
- acute headache, nausea, and vomiting (which are the signs of life-threatening conditions such as stroke).
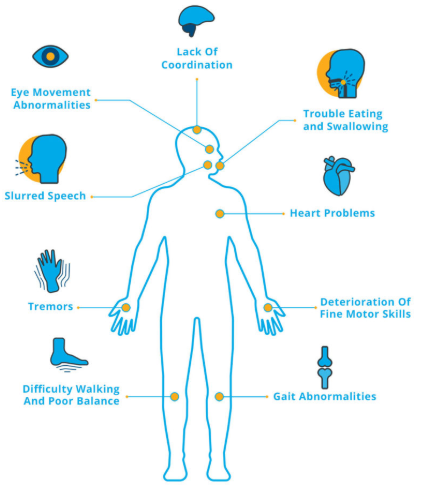
In most cases, symptoms of ataxia get worse with time, significantly deteriorating the quality of life and normal functioning of the patient.
Types of Ataxia
Ataxias may be inherited, acquired or idiopathic (if the cause remains unknown).
Inherited Ataxia
Inherited ataxia includes dozens of rare heterogeneous neurological diseases, the most frequent types of which are spinocerebellar ataxias, Friedreich ataxia and Ataxia telangiectasia. They are caused by a failure in one or multiple genes and are passed on through generations.
Acquired Ataxia
Acquired ataxia is caused by damage to the brain or spinal cord due to disease, injury, or exposure to alcohol or certain drugs.
Idiopathic Ataxia
Idiopathic ataxia includes all ataxia with unknown causes. Also called cerebellar ataxia.
Modern Approaches to Ataxia Treatment
When the treatment of the underlying disease is available, the symptoms of ataxia may be completely or partly cured and/or progression of the disease may halt. For example, in the case of resectable tumors and vitamin and coenzyme deficiencies, patients recover in 6 months to a year after the underlying cause is treated.
Medical scientists are still searching for the most effective treatment to prevent onset or inhibit progression of most types of ataxia.
Several clinical ongoing studies showed partial improvement of ataxia symptoms in patients with spinocerebellar ataxia (SCA) treated with lithium or varenicline 12. Improvement was also seen when using riluzole in patients with cerebellar ataxia of different etiologies.
Symptomatic treatment is used to relieve muscle spasms, tremors, bladder problems, abnormal eye movements and depression, as well as for cardiac problems seen in Friedreich’s ataxia. Other dysfunctions such as fatigue and neuropathy are also managed with the relevant medications.
Physiotherapy and physical exercises play a significant role in increasing quality of life as they may help to preserve mobility and increase muscle strength. Speech and language therapists can help to improve speaking and communication problems, difficulties with swallowing, coughing and choking. An occupational therapist can also be helpful, for example, with home adaptations, teaching strategies for daily activities or wheelchair assessments.
Beside that, treatment with cell products is currently considered as a promising approach in ataxia.
Contact us
Get a free online consultation from one of our Medical Advisors to find out if stem cells would work for you.

Medical Advisor, Swiss Medica doctor
Scientific Evidence of Stem Cell Therapeutic Effect on Ataxia
Recent studies demonstrate that stem cell treatment is easily tolerated and can provide remarkable improvements in patients.
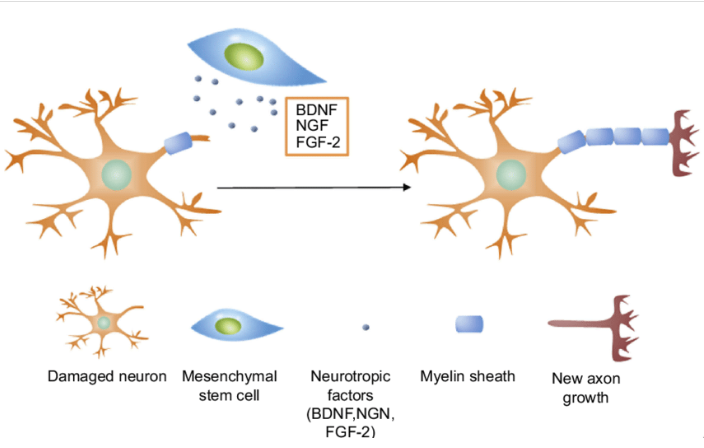
It is assumed that degenerating neurons attract injected multipotent mesenchymal stem cells (MSCs) and connect with them. The MSCs then directly provide neuronal growth factors to cells that are being destroyed to rescue them from degeneration. It was also shown that these types of cells induce synaptic connections and reduce apoptosis in neurons. For example, a single injection of MSCs in mice with spinocerebellar ataxia positively affected the progressive degeneration of both axons and myelin in the spinal cord.
Expected Improvements
Due to neuroprotective, neurotrophic, anti-inflammatory effects of MSCs and the ability of this cell product to stimulate neovasculature formation, the clinical effect in patients suffering from ataxia includes:
- Improvements of coordination and balance;
- Increased strength in the limbs and muscles;
- Improvement of fine motor skills and speech;
- Reduction of fatigue;
- Overall increased quality of life.
Results of Swiss Medica Patients
Swiss Medica offers complex neurological rehabilitation for patients with ataxia. The causes of ataxia are diverse and, therefore, a customized approach is created for each patient.
Feedback from a post-stroke patient treated with stem cells:
“I had absolutely no expectations. But after two days of treatment, small miracles began to happen. The spasticity in my left leg disappeared, the strength of my left hand equated to my right hand, no more weakness in my left hip, my walking improved by 70%. What I am most looking forward to is to return to Australia and going back to my doctors and neurologists who told me that stem cell treatment for strokes was a waste of time. I’d love to look at their faces when I walk in”.
Relapsing-Remitting Multiple Sclerosis treatment. “I’ve got my life back. And now I am 100% better than I was this time last year. I can walk my son to school. I can do things that I couldn’t do. Now I’m swimming 22 lengths twice a week. I just feel brilliant and I’m so excited”.
Patient from Australia.
Diagnosis: Stroke in the medulla.
Duration: 4 years.
Before the treatment:
- Weakness at the left-hand side of the body;
- Unable to walk.
After the treatment:
- No spasticity and weakness in the limbs;
- Walking improved by 70%
Feedback from a patient with multiple sclerosis:
“I have booked snowboarding holidays, so I am hoping that my leg will be strong enough to take me to the Alps and back”.
Patient from the UK.
Diagnosis: Multiple sclerosis.
Duration: 4 years.
Before the treatment:
- Walked with a stick;
- Could not walk far;
- Poor balance;
- Hard to pick things up with right arm;
- Severe fatigue.
After the treatment:
- Walking improved considerably;
- No fatigue – able to exercise normally;
- No longer stumbles while speaking;
- Right arm is active again;
- Restored strength in limbs.
More video testimonials are available on our YouTube channel.
Ataxia Treatment with Stem Cells: the Procedure
The therapy of ataxia at Swiss Medica includes intrathecal and/or intravenous administration (injection into the spinal canal and/or through an IV Drip) of autologous (patient’s own) bone marrow cells combined with placental cells and umbilical cord cells. The procedure of collecting cells from a patient is preceded with local anesthesia.
 |  |
| A | B |
Cells harvested from the patient’s bone marrow undergo several steps prior to administration: they are processed, separated using a centrifuge, cultivated if required, and bioactivated to get specific cell products for optimal effects. Donor cell-based products are already prepared and can be introduced immediately.
Cells are then delivered into the body intrathecally. The complete treatment process in the clinic lasts about 10 days and the effect may be observed within 15-40 days. It is recommended to repeat the course after 6 months to maintain the results and get better improvements.
Indications and Contraindications for Stem Cell Therapy
Stem cell therapy may be beneficial to those patients who suffer from diseases or medical conditions accompanied by symptoms of ataxia. These are:
- multiple sclerosis;
- post-stroke;
- Parkinson’s disease;
- head injury;
- sarcoidosis;
- celiac disease;
- toxic reaction,
- and others.
However, there are some limitations that can hinder the positive effects of stem cell treatment or are considered direct contraindications, such as any life-threatening or terminal health conditions (including cancer or tumors) and past negative experience with cell products. Other contraindications for stem cell therapy are:
- infectious disease in the acute stage;
- stroke or transient ischemic attack in the last 3 months;
- deviations of some indicators in blood tests;
- pregnancy and lactation;
- mental disorders and addictions;
- contraindications to anesthesia and/or high risk of bleeding and/or pathological processes in the area of the proposed biopsy (does not exclude the possibility of using donor cell products) and some others.
Safety of Stem Cell Therapy and Possible Side Effects
Safety of stem cell treatment has been shown in several clinical studies in patients with different pathologies. Most patients tolerate the procedure well. Short-term and transient fever during the procedure, while rare, cannot be excluded. Swiss Medica specialists will monitor your condition for safer and more beneficial results.
What the Treatment Includes
Due to a complex factor standing behind each case of ataxia, additional therapies may be required. The patient’s neurological status, main diagnoses, age, family history and other aspects are considered when the individual therapy plan is developed. Swiss Medica offers potent options to improve results of stem cell treatment in patients suffering from ataxia. The following procedures are advised for such patients:
- Kinesiotherapy;
- Electrical myostimulation;
- Interval Hypoxic Hyperoxic Treatment;
- Mesodiencephalic modulation (transcranial electrostimulation of the brain).
Consultations of specialist of interfacing areas (neurorehabilitation, psychology, and nutrition) are also available.