The Stromal Vascular Fraction (SVF) is the co-product of fat (adipose) tissue processing. Treatment with the SVF cells is an advanced approach in the stem cell therapy used in patients with various autoimmune, urologic, neurologic, pulmonary, ophthalmologic and orthopedic diseases. The SVF contains several different stem cell types, as well as growth factors and other biologically active components. Isolation of the SVF cells from fat tissue is a rather simple process. The complete process, including pre-anesthesia, isolation and the injection of SVF cells only takes about 4 hours and is performed in a clinic. The procedure is usually well tolerated and has been clinically confirmed as safe.
What is SVF
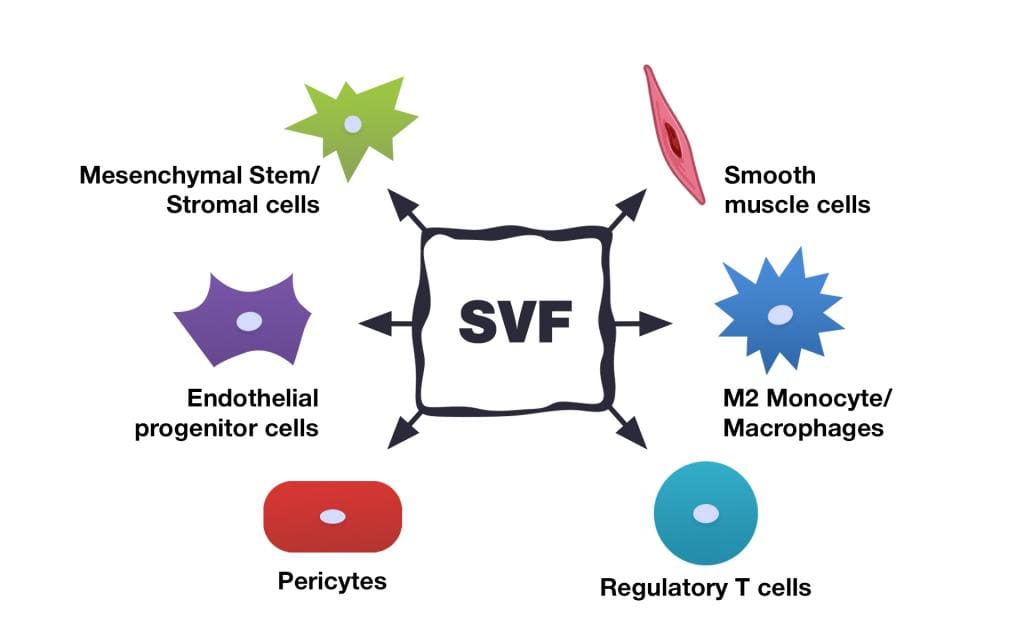
The Stromal Vascular Fraction (SVF) is the co-product of fat (adipose) tissue processing. The content of SVF includes several stem cell types which are precursors of fat tissue cells, as well as immune cells, fibroblasts, pericytes, endothelial cells, and others. The SVF also contains growth factors and other biologically active components. All of these factors have the potential to provide therapeutic benefits, which is why the SVF gained attention so rapidly among specialists in stem cell treatments.
Treatment with the SVF cells is an advanced approach in stem cell therapy. Isolation of the SVF cells from fat tissue is a rather simple process which takes approximately 30–90 min. It utilizes a traditional technique of liposuction and is performed in a clinical setting within half of a day. These stem cells can be introduced directly into the damaged areas by the means of a minimally invasive technique.
Patients who benefit from an SVF-based approach
The adipose SVF has practical clinical application in the treatment of such common diseases and conditions as:
- Rheumatoid arthritis;
- Joint replacement;
- Osteoarthritis;
- Soft tissue injury;
- Ligament injury;
- Migraine and tension headaches;
- Diabetes;
- Chronic ischemic cardiomyopathy;
- Hair loss;
- Erectile dysfunction;
- Pulmonary diseases;
- Autoimmune diseases (multiple sclerosis, lupus, Crohn’s disease, etc.).
It is also beneficial in certain neurological conditions, such as neuropathy, ALS, and Parkinson’s disease. Several clinical applications of adipose-derived stem cells are currently under investigation.
How does SVF work?
SVF cells are involved in tissue regeneration and healing through a variety of mechanisms. It is supposed that they secrete cytokines, chemokines, and growth factors which stimulate tissue reparation processes. An important part of their mechanism of action is that SVF is a heterogeneous cellular product. It consists of various cell populations that ensure the presence of target cells in the product, which are affected by cytokines produced by stem cells. For example, one of the main effects of SVF is to stimulate the formation of new blood vessels (neoangiogenesis). This very quick process (3 to 10 days) is based on the ability of angiogenic cytokines to immediately find the target endothelial and smooth muscle cells of SVF.
What does the treatment involve?
The basic steps of the treatment with the SVF are:
- Administration of anesthesia (general or local);
- Cell harvesting via lipoaspiration;
- Cell processing to obtain stromal vascular fraction;
- Injection of the SVF into the bloodstream or locally into the treated organ. Cells of the SVF are used immediately after harvesting.
What happens during the procedure?
The patient is transferred to the operating room where a Global Power LLC doctor administers anesthesia. When the effect of the anesthesia is achieved, the doctor makes a small incision in an area of the body with fatty tissue, such as the buttocks or abdomen. The patient’s own fat tissue is gently removed with a special syringe. The obtained solution, which is called lipoaspirate, is processed with a collagenase solution (it destroys the extracellular matrix and releases the cells from the fat tissue), and then is subjected to centrifugation to separate the SVF. Finally, the solution of SVF is administered to the patient. Depending on the clinical procedure, the patient may be monitored for some time in the Global Power LLC clinic.
Preliminary procedures
Prior to the procedure, the doctor in Global Power LLC examines you, determines your current health state and individual characteristics, collects information on your complete medical history, reveals leading clinical symptomatology and carries out laboratory tests. In the operating room, the doctor prepares your skin by cleaning it with an antiseptic and then administers a twilight anesthetic or general anesthesia.
Duration
The complete procedure, starting from the administration of an anesthetic to the moment when the patient may leave the clinic, takes only about 4 hours. The processing of adipose-derived SVF cells takes somewhere between 75–90 minutes, depending on the amount of tissue aspirated from the patient. The patient is then monitored to ensure his or her safety.
Your comfort during the procedures
You may feel some discomfort similar to the sensations from ordinary syringe injections while the doctor introduces the processed cells into the designated location.
Basic difference between multipotent MSCs (mesenchymal stromal cells) and SVF
Multipotent MSCs are the most studied and characterized cell line that is present in SVF. Mesenchymal stem cells are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts (bone cells), chondrocytes (cartilage cells), myocytes (muscle cells) and adipocytes (fat cells which give rise to marrow adipose tissue). Adipose tissue is a rich source of multipotent MSCs. However, laboratory cultivation in special sterile conditions according to the specific standards is needed to obtain a cell product containing multipotent MSCs that will be ready for use in treatments. Whereas SVF cells may be used within 2-3 hours after lipoaspiration and do not need cultivation, which reduces the risk of contamination and possible changes to the cells’ properties.
SVF has all the benefits of multipotent MSCs, while also having a number of other valued effects derived from the presence of other stem cell lines and active molecules.
Main indications of multipotent MSCs treatment
Multipotent MSCs are commonly used for the treatment of the following diseases:
- Skin burns;
- Fractures;
- Autoimmune diseases;
- Transplant against host reaction;
- Chronic limb ischemia and others.
Therapeutic effect
The therapeutic potential of multipotent MSCs is based on their ability to produce biologically active molecules (cytokines and growth factors) which are involved in such regenerative processes as:
- The growth of new blood vessels and nerve terminals;
- Inflammation control;
- Programmed cell death suppression;
- The attraction of stem cells to the location of the pathological process;
- Induction of stem cell differentiation.
Difference in treatment procedures
Treatment with multipotent MSCs requires cell cultivation in special laboratory conditions (a culture medium with additional nutrients for growth and proliferation, sterility and qualified personnel, as well as facilities to carry out this work). When treatment with adipose-derived stromal vascular fraction is performed, the lipoaspirate is processed within 2-3 hours using equipment which can be located directly in the operating room. There is no need to maintain sterile conditions and follow complicated laboratory standards of cell cultivation.
Safety of the SVF: Risks and side effects
Clinical trial results indicate that local injection of SVF has shown a safe profile, with no risk of neoplastic formation, unwanted tissue differentiation or adverse effects. The procedures are usually well tolerated in the majority of patients. Individual intolerance, however, rarely occurred but cannot be excluded. Global Power LLC specialists will monitor your condition for safer and more beneficial results and will take appropriate measures to mitigate any possible risks.